Extend the Shelf Life of Your Food
Oxygen absorbers are small packets that remove oxygen from their surroundings. When used in airtight containers, they reduce the oxygen content and extend the shelf life of food.
How Oxygen Absorbers Work
These packets contain substances that react with and bind oxygen. A 50cc absorber can remove at least 50 ml of oxygen, often even more. An indicator called "OxyEye" changes color to show whether the absorber is still active.
Applications
Oxygen absorbers are used in various areas:
- Food industry: To preserve food and extend shelf life.
- Antique preservation: Protecting delicate materials from oxidation.
- Long-term storage: Preserving staples, clothing, documents, and more.
Calculation Example: How Many Oxygen Absorbers Are Needed?
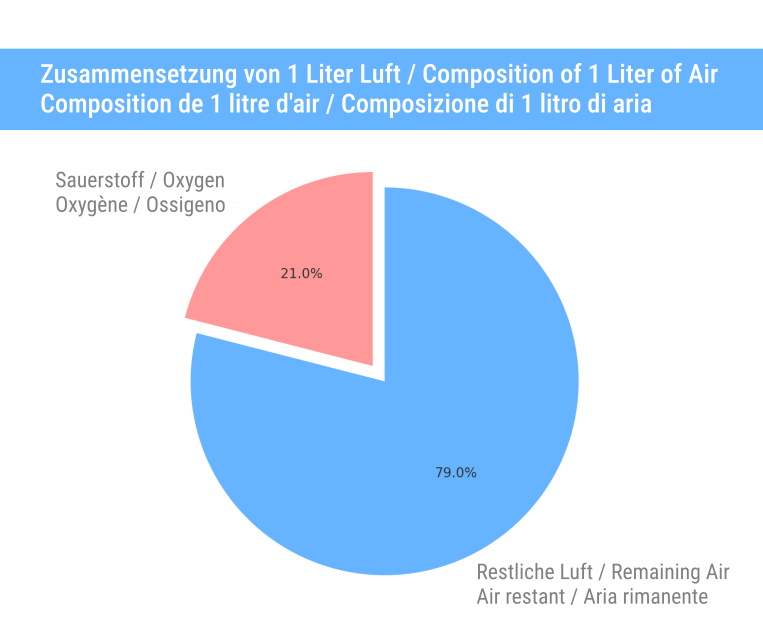
Suppose you want to protect 1 liter of volume in an airtight bag using oxygen absorbers:
- Air contains approximately 21% oxygen, which corresponds to 210 ml of oxygen in 1 liter (1000 ml) of air.
- A single oxygen absorber with a capacity of 50cc can bind 50 ml of oxygen.
To remove the entire volume of 210 ml of oxygen, you need:
210 ml ÷ 50 ml = 4.2 oxygen absorbers
Therefore, you should use 5 oxygen absorbers to ensure no oxygen remains.
Usage Tips
For optimal results, oxygen absorbers should be used in combination with food-grade aluminum composite bags. These bags offer additional protection against moisture and light. Further instructions on use can be found in our guides for long-term storage.
By using oxygen absorbers, you can effectively improve the quality and shelf life of your supplies.



